-
Once deemed heretical, the idea of “inheritance of acquired characteristics” is now gaining evidence from multiple species, including mammals. Studies from our lab and others now promote the idea that paternally acquired phenotypes (e.g. metabolic disorders) from environmental stressors can be encoded in the form of sperm RNAs & RNA modifications as a sperm 'RNA code' (Nat Rev Endocrinol 2019, 2020) which transmit paternal phenotypes to the offspring via shaping early embryo development (Nat Rev Genet 2016; Nat Rev Mol Cell Biol 2018)
tRNA-derived small RNAs (tsRNAs) and rRNA-derived small RNAs (rsRNAs) are newly discovered small non-coding RNAs with diverse functions. As critical components of the 'sperm RNA code', we showed that sperm tsRNAs/rsRNAs can act as hereditary factor that contribute to the intergenerational transmission of paternally acquired metabolic disorder. Read more



embryo symmetry breaking
sperm RNA code
During mammalian early embryo development, how do different lineages emerge? Are 2-cell blastomeres absolutely equal? If not, can there be even small differences at molecular levels? and if so, how do they arise at the first place and how could they get amplified to guide future cell fate? Read more
We use novel tools including SPORTS, PANDORA-seq and mass spectrometry-based methods such as LC-MS/MS and MLC-seq to study how small RNAs derived from fragmentation of longer parental RNAs (e.g., tRNAs, rRNAs), along with their RNA modifications and responsible enzymes, dynamically regulate gene expression and cause diseases such as cancer. Read more
the RNA 'renovatio'
Latest Lab news (More news)
2025.2 We publish a two-stage activation hypothesis explaining how abnormal small RNA biogenesis spark autoimmune diseases via small RNA-TLR7/8 interactions in Trends in Biochemical Sciences
2024.11 Qi gave a talk at the tRNA conference 2024, showing how RNA modifications reshape tsRNA structure and function.
2024.9 Qi gave a keynote lecture at the EMGS 2024 meeting, discussing the function of sperm RNA code and emerging tools to decode it.
2024.9 Glad to publish with Shenglong Zhang lab on Mass Spectrometry-Based direct Sequencing of RNAs in Journal of the American Chemical Society, which enables de novo and quantitative mapping of multiple RNA modifications. Exciting times ahead.
2024.8 Our collaborative work with Bruno Di Stefano lab on P-body RNAs and myeloid leukaemia is published in Nature Cell Biology
2024.8 We have our NIH/NICHD R01 grant renewed, will continue exploring RNA and RNA modification-mediated epigenetic inheritance
2024.6 We discuss how sperm mitochondrial tsRNAs sense paternal diet and influence son's health through embryo development in Nature; Qi did a interview in Nature about the momentum of sperm RNA-mediated epigenetic inheritance research & our previous work in Science
2024.4-5 Qi gave seminar at University of Florida, and conference talks at ASA 2024 and NATW 2024, forging new collaborations.
2024.3 Qi gave talks at SOT 2024 and SRI 2024 conferences, great to connect with old and new friends.
2024.3 We publish with Xuemei Chen lab on the discovery of proteins controlling NAD+ cap of RNAs in Nature Communications, there are so many more to discover in RNA modifications & termini.
2023.11-2024.1 Qi gave seminars at UCSC, Caltech and The Scripps Research Institute on RNA-mediated epigenetic inheritance and the emerging new tools to study the expanding universe of small RNAs
- We explore the expanding universe of small RNAs with new tools including SPORTS (GPB 2018), LC-MS/MS (Science 2016), PANDORA-seq (Nat Cell Biol 2021) & MLC-seq (J Am Chem Soc 2024); decoding the 'RNA code' (RNA expression & modification profiles) in regard to evolutionary origins and functional principles, and explore how the sperm RNA code influence embryo development such as by regulating cell fate decision (Development 2015; Nat Commun 2018; Cell 2018) that contribute to offspring health.
-
We pioneered the systematic discovery of tRNA-derived small RNAs (tsRNAs) (Cell Res 2012, Science 2016) & rRNA-derived small RNAs (rsRNAs) (Nat Cell Biol 2018, Nat Cell Biol 2021) in mature sperm; and that sperm tsRNAs/rsRNAs, along with their RNA modifications, act as epigenetic factors in mediating intergenerational inheritance of acquired metabolic disorders, which require the action of RNA methyltransferase Dnmt2 (Science 2016, Nat Cell Biol 2018)
- Our discovery of abundant tsRNAs in physiological contexts such as sperm and serum, coupled with their sensitive responses to environmental stimuli and diseases, has sparkled a new wave of tsRNA research. These tsRNAs, which are evolutionarily ancient (predating miRNAs), harness their linear sequences and 3D structures to perform unexpected biological functions (Trends Biochem Sci 2021, J Biol Chem 2023), which embodies the concept of tRNA ‘renovatio’ (from Latin, meaning renewal and rebirth) (Mol Cell 2023). The artwork depicts the re-creation process of tsRNAs through the metaphor of a supernova explosion, in which the end of one stellar life cycle triggers the genesis of new stars.
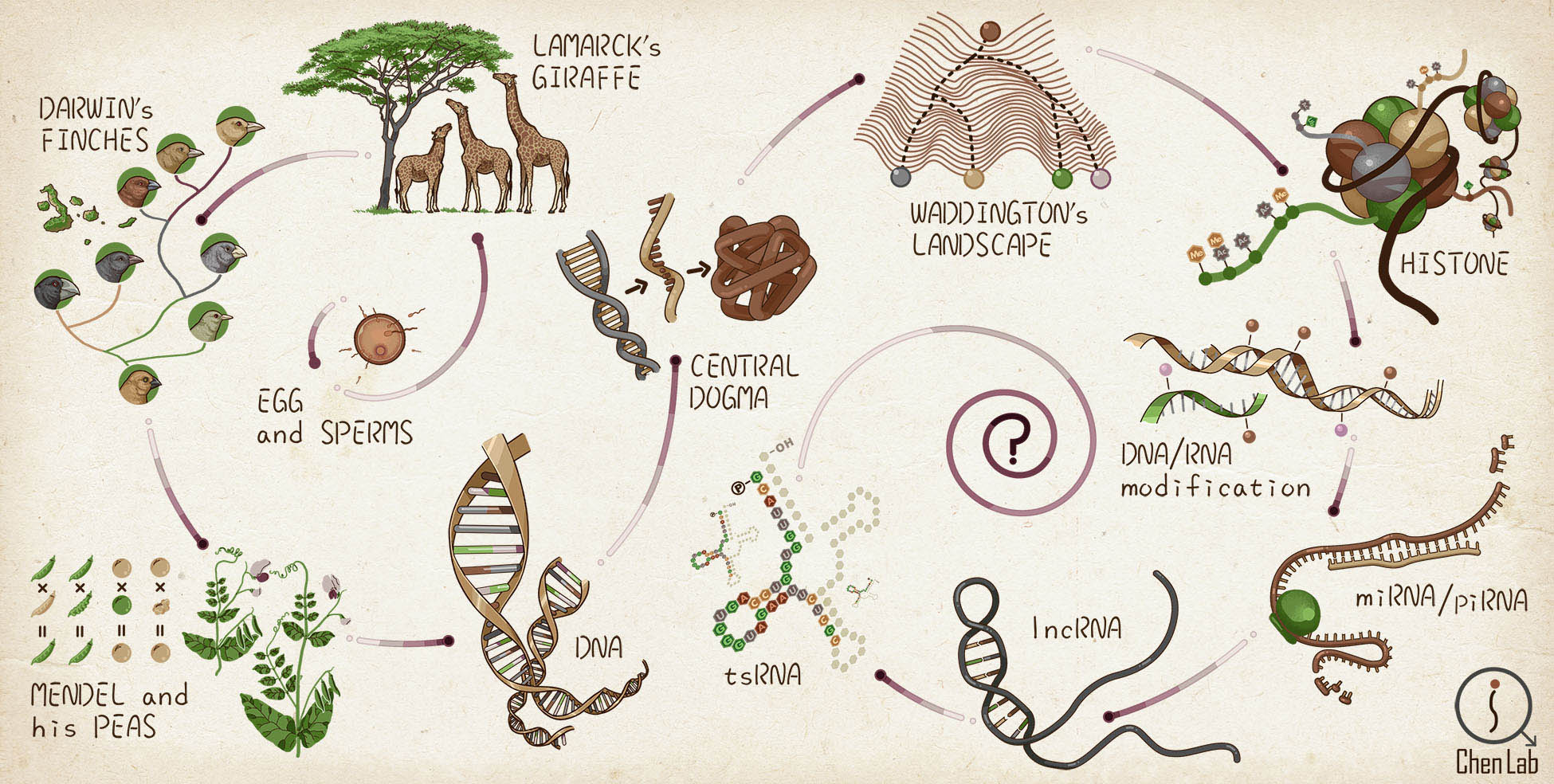
The Evolving Ideas of Heredity & Hereditary Information Carriers (further read)
Exploring the small RNA universe with new tools (futher read)
- The idea of RNA ‘renovatio’ has been further accentuated by our new method PANDORA-seq (Nat Cell Biol 2021), which systematically reveals abundant existence of various types of small noncoding RNAs (sncRNAs) beyond the siRNAs/miRNA/piRNAs, including sncRNAs derived from various parental RNAs (tRNA, rRNA, snoRNA, snRNA, Y RNA, Vault RNA etc) carrying different termini and RNA modifications that are previously undetectable by using traditional small RNA-seq. This has redrawn the boundaries of the small RNA universe, heralding new directions in research (Nat Cell Biol 2022)